UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): |
(Exact name of Registrant as Specified in Its Charter)
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
||||
|
||||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
|
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure
On May 8, 2023, Sight Sciences, Inc. (the “Company”) posted an investor presentation to its website at https://investors.sightsciences.com/. The Company expects to use the investor presentation, in whole or in part, and possibly with modifications, in connection with presentations to investors, analysts, and others. A copy of the investor presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K.*
Item 9.01 Financial Statements and Exhibits
(d) Exhibits
Exhibit No. |
Description |
99.1 |
|
104 |
Cover Page Interactive Data File, formatted in Inline XBRL. |
* |
The information in Item 7.01 and Exhibit 99.1 of this Current Report on Form 8-K shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
Sight Sciences, Inc. |
|
|
|
|
Date: |
May 8, 2023 |
By: |
/s/ Paul Badawi |
|
|
|
President and Chief Executive Officer |

Delivering the Power of Sight Investor Presentation May 2023 ® ™

Forward-Looking Statements This presentation, together with other statements and information publicly disseminated by the Company, contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which statements are subject to considerable risks and uncertainties. The Company intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements other than statements of historical fact, including statements regarding our future results of operations, product development, market opportunity, clinical trial results and timeline, and business strategy and plans. These forward-looking statements include, but are not limited to, statements concerning the following: the Company's projected financial results, including revenue and revenue guidance; estimates of the Company’s total addressable market; the Company’s ability to enter into and compete in new markets; the Company’s ability to successfully develop and commercialize its product pipeline; the Company’s ability to compete effectively with existing competitors; the Company’s ability to manage and grow its business by expanding its sales to existing customers or introducing our products to new customers; the Company’s ability to successfully execute its clinical trial roadmap so as to achieve its strategic objectives; the Company’s ability to successfully execute its strategic initiatives and objectives; and the Company’s ability to obtain and maintain sufficient reimbursement for its products. These statements often include words such as “anticipate,” “expect,” “suggests,” “plan,” “believe,” “intend,” “estimates,” “targets,” “projects,” “should,” “could,” “would,” “may,” “will,” “forecast” and other similar expressions. Management bases these forward-looking statements on its current expectations, plans and assumptions affecting the Company’s business and industry, and such statements are based on information available as of the time such statements are made. Although management believes these forward-looking statements are based upon reasonable assumptions, it cannot guarantee their accuracy or completeness. Forward-looking statements are subject to and involve risks, uncertainties and assumptions that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance, or achievements predicted, assumed or implied by such forward-looking statements. Some of the risks and uncertainties that may cause actual results to materially differ from those expressed or implied by these forward-looking statements are discussed under the caption “Risk Factors” in the Company’s filings with the U.S. Securities and Exchange Commission, as may be updated from time to time in subsequent filings. These cautionary statements should not be construed by you to be exhaustive and are made only as of the date of this press release. The Company undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law. Certain information contained in this presentation relates to, or is based on, studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its own estimates and research are reliable, such estimates and research have not been verified by any independent source. The Company has proprietary rights to trademarks, trade names and service marks appearing in this presentation that are important to our business. Solely for convenience, the trademarks, trade names and service marks may appear in this presentation without the ® and ™ symbols, but any such references are not intended to indicate, in any way, that we forgo or will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, trade names and service marks. All trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. The Company does not intend its use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of the Company by, these other parties. Without limitation, SIGHT SCIENCES™, SIGHT SCIENCES (with design)®, OMNI®, SION™, TEARCARE® , SMARTLIDS™ and DELIVERING THE POWER OF SIGHT ™ are trademarks of Sight Sciences, Inc. in the United States and other countries. 1 ®

2 To transform the treatment of Glaucoma and Dry Eye through a broad portfolio of innovative solutions Our solutions are designed for earlier intervention and to help restore the natural functionality of healthy eyes Our Mission Eye Care Innovation in Glaucoma and Dry Eye
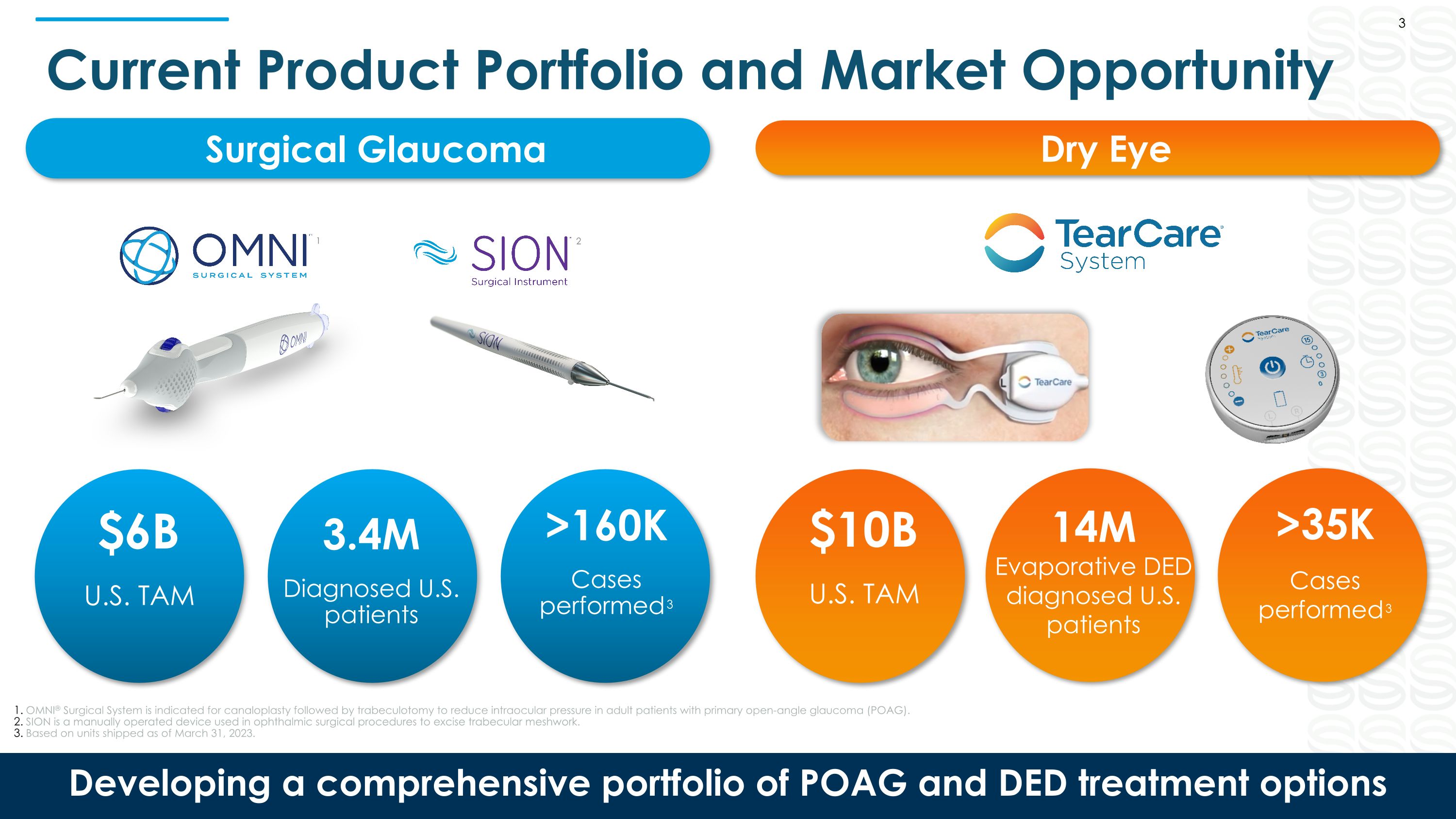
Current Product Portfolio and Market Opportunity Developing a comprehensive portfolio of POAG and DED treatment options 3.4M Diagnosed U.S. patients $6B U.S. TAM 14M Evaporative DED diagnosed U.S. patients $10B U.S. TAM >160K Cases performed3 >35K Cases performed3 OMNI® Surgical System is indicated for canaloplasty followed by trabeculotomy to reduce intraocular pressure in adult patients with primary open-angle glaucoma (POAG). SION is a manually operated device used in ophthalmic surgical procedures to excise trabecular meshwork. Based on units shipped as of March 31, 2023. 3 Surgical Glaucoma Dry Eye 2 1

Strategic Value-Creation Initiatives Bedrock of Clinical Excellence: numerous completed, ongoing and planned trials in POAG and DED 4 Train New OMNI Surgeons Expand OMNI Usage in Standalone and Combination Cataract MIGS Procedures SURGICAL GLAUCOMA Extend Portfolio by introducing SION for certain customer subsegments Pursue Reimbursement for TearCare Procedures SAHARA RCT: Validate Health and Economic Value Proposition for TearCare® against the Market Leader in Dry Eye Treatment DRY EYE

We believe OMNI is singularly well-suited to address all three primary points of resistance Underlying POAG Diseased Anatomy TRABECULAR MESHWORK SCHLEMM’S CANAL COLLECTOR CHANNELS Trabecular Bypass Stents Canaloplasty Only Trabeculotomy Only 1 2 3 1 2 3 TRABECULAR MESHWORK SCHLEMM’S CANAL DISTAL COLLECTOR CHANNELS Canaloplasty using OMNI expands and dilates Schlemm’s canal and collector channels Three primary points of resistance in the conventional outflow pathway Trabeculotomy using OMNI unroofs the trabecular meshwork 5

6 OMNI: Interventional POAG Procedure Two sequential, ab interno MIGS procedures to help restore natural drainage in the eye Up to 360° coverage with a single clear corneal microincision Trabeculotomy using OMNI Canaloplasty using OMNI Schlemm’s Canal Schlemm’s Canal Trabecular Meshwork Trabecular Meshwork Collector Channels Cannula Tip Cannula Tip Microcatheter Microcatheter Clear Corneal Microincision Clear Corneal Microincision Collector Channels

Market Leading Label Allows Broad Use in POAG PRESCRIPTION MEDICATIONS STANDALONE (>85%1 of POAG; ~5.4M eyes) COMBINATION CATARACT (<15%1; ~0.8M eyes) CONVENTIONAL TREATMENTS TRABECULAR BYPASS STENTS LASER TRABECULOPLASTY CONVENTIONAL SURGERY MILD (~40%) MODERATE (~40%) ADVANCED (~20%) Represents internal analysis of third-party estimates for % of U.S. POAG patients. 1 1 ALTERNATIVE BYPASS STENTS MIGS GONIOTOMY 1 7

OMNI: Customizable to All Six MIGS Categories in POAG2 Low Risk of Hyphema Consistency of Efficacy Degree of Efficacy Represents internal analysis of third-party estimates for % of U.S. POAG TAM. The described procedures are presented for illustrative purposes only and are based on observed physician practice. Mild Disease (40%)1 Moderate Disease (40%)1 Advanced Disease (20%)1 Primary Distinguishing Treatment Requirements for MIGS Procedures: 360° Canaloplasty + 45° - 90° Trab or Goniotomy 360° Canaloplasty + 90° - 180° Trab 360° Canaloplasty + 90° - 180° Trab 360° Canaloplasty + 180° Trab 360° Canaloplasty + 180° - 360° Trab 360° Canaloplasty + 360° Trab Combination Cataract MIGS <15%1 Standalone MIGS >85%1 8 $1B TAM $5B TAM

AAO IRIS® Registry PRECISION Database of over 483 million real-world visits by 78 million de-identified patients submitted by ophthalmologists Compares OMNI, iStent® and Hydrus® in combination with cataract surgery Two year IOP reduction and medication usage Plan to publish in peer-reviewed journal by end of 2023 Additional IRIS® studies in development, including Standalone MIGS with OMNI First randomized clinical trial comparing a MIGS technology versus standard of care hypotensive medication Pseudophakic mild-to-moderate POAG N > 200, randomized 2:1 / OMNI : prostaglandin analog 12-month endpoint Plan to begin enrollment 4Q23 OMNI® Clinical Roadmap 9 Note: Clinical trials, including their design, endpoints and timing, are subject to change at the Company’s discretion. Initial results may include preliminary data and interim analyses that are subject to change. Executing Groundbreaking Clinical Trials

Complement to OMNI for Certain Customer Subsegments 10 Fully meets AAO definition of goniotomy, aligns with Category I CPT code 65820 Innovative design bladelessly excises diseased trabecular meshwork within several clock-hours SION: Bladeless Goniotomy Procedure
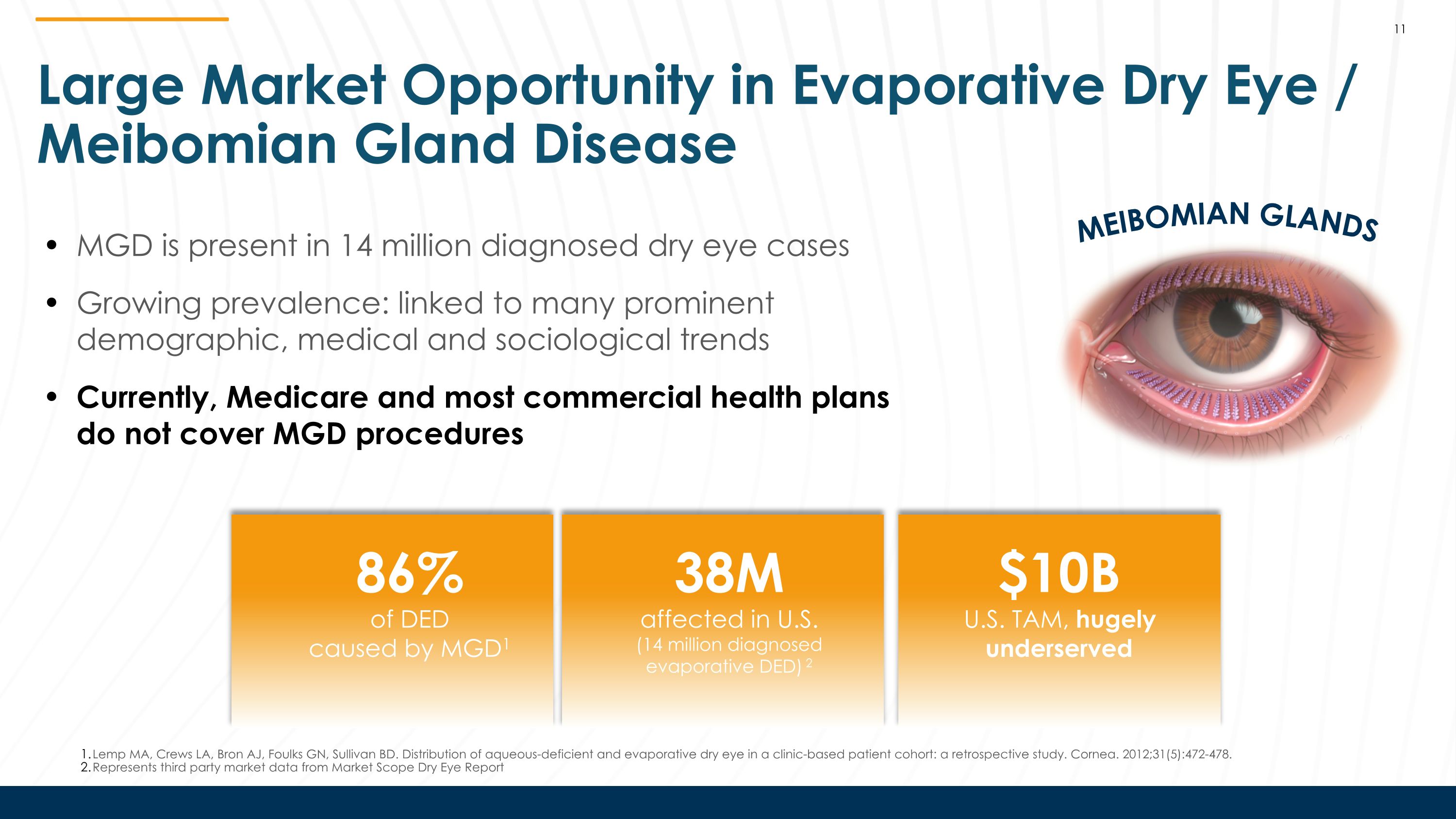
Large Market Opportunity in Evaporative Dry Eye / Meibomian Gland Disease MEIBOMIAN GLANDS MGD is present in 14 million diagnosed dry eye cases Growing prevalence: linked to many prominent demographic, medical and sociological trends Currently, Medicare and most commercial health plans do not cover MGD procedures 38M affected in U.S. (14 million diagnosed evaporative DED) 2 86% of DED caused by MGD1 $10B U.S. TAM, hugely underserved 11 Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478. Represents third party market data from Market Scope Dry Eye Report

Our Solution: TearCare The only wearable eyelid technology designed to melt and remove meibomian gland obstructions TearCare Technology Steady temperature and precise heat delivery Single-use SmartLids™ conform to variable eyelid anatomy while allowing natural blinking Designed for intuitive provider training and comfortable patient experience Expect 1 to 2 treatments per patient per year Eyelid Therapy for Evaporative Dry Eye MGD: hardened meibum forms obstructions, reducing tear quality leading to premature evaporation Liquefying meibum requires precise (40-42° C at the inner eyelid) and consistent (15 minutes) heat1 Manual expression clears glands 12 Blackie CA, Solomon JD, Greiner JV, Holmes M, Korb DR. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci. 2008 Aug;85(8):675-83. doi: 10.1097/OPX.0b013e318181adef. PMID: 18677234.

The SAHARA RCT Note: Clinical trials, including their design, endpoints and timing, are subject to change at the Company’s discretion. Initial results may include preliminary data and interim analyses that are subject to change. 2021 First patient, first visit 2Q 2021 2022 Enrollment completed 3Q 2022 2023 6-month superiority endpoint: plan to release topline results summer 2023; publish and present full results Q4 2023 2024-25 12-month results expected 2H 2024 24-month results expected 2H 2025 13 SAHARA RCT (ongoing) Head-to-head vs. market leading DED Rx eyedrop Multi-center U.S. RCT; enrollment complete 24-month study period (n = 300) Goal: demonstrate safety and effectiveness of TearCare® procedures compared to Restasis® to treat the signs and symptoms of dry eye disease in adult patients Six-month period to study superiority to 2x / day use of Restasis® Restasis arm receives TearCare treatment and 6-month follow-up TearCare arm: 24-month durability study period Primary outcome measures: tear break-up time, OSDI score Designed to drive reimbursement with input from eight payor medical directors

Develop Comprehensive Best-in-Class Portfolio SURGICAL GLAUCOMA PIPELINE MILD TO MODERATE DISEASE In-office Injection of Sustained Release Pharmaceutical (Rx)* 1 MILD TO MODERATE DISEASE OR Performed Canal-based Glaucoma Surgery (MIGS) MODERATE TO ADVANCED DISEASE OR Performed Suprachoroidal Implant (MIGS)* 5 MILD TO EARLY MODERATE DISEASE Implantable Canalicular Scaffold (MIGS)* 2 MILD TO EARLY MODERATE DISEASE Goniotomy 3 14 *This pipeline product is under development and is not commercially available 4 COMMERCIALLY AVAILABLE

Over-the-counter Artificial Tear With A Differentiated Lipid Layer Technology* Dry Eye Disease Prescription Pharmaceutical Eyelid Ointment* Office-Based Eyelid Procedure Home-Based Eyelid Device Treatment* 1 2 4 Develop Comprehensive Best-in-Class Portfolio DRY EYE PIPELINE 3 15 *This pipeline product is under development and is not commercially available

Strong Financial Profile 25 Annual Revenue FY18-FY22 CAGR: +76% Quarterly Revenue Q1’23 Growth: +26% vs. Q1’22 FY22 Revenue: $71.3M, +46% Surgical Glaucoma: $65.6M, +41% Dry Eye: $5.7M, +133% ($ Millions) ($ Millions) $18.8 $14.9 $17.2 $18.7 $20.5 $7.5 $23.3 $27.6 $49.0 $71.3 16 Balance Sheet $167.3M cash and cash equivalents and $35M long-term debt* as of 3/31/23 2023 Revenue Guidance $89M to $94M, +25% - 32% growth compared to 2022 Gross Margin 84% Gross Margin Q1 2023 83% Gross Margin in 2022 *Total long-term debt defined as debt outstanding under our term loan agreement before debt discount.
